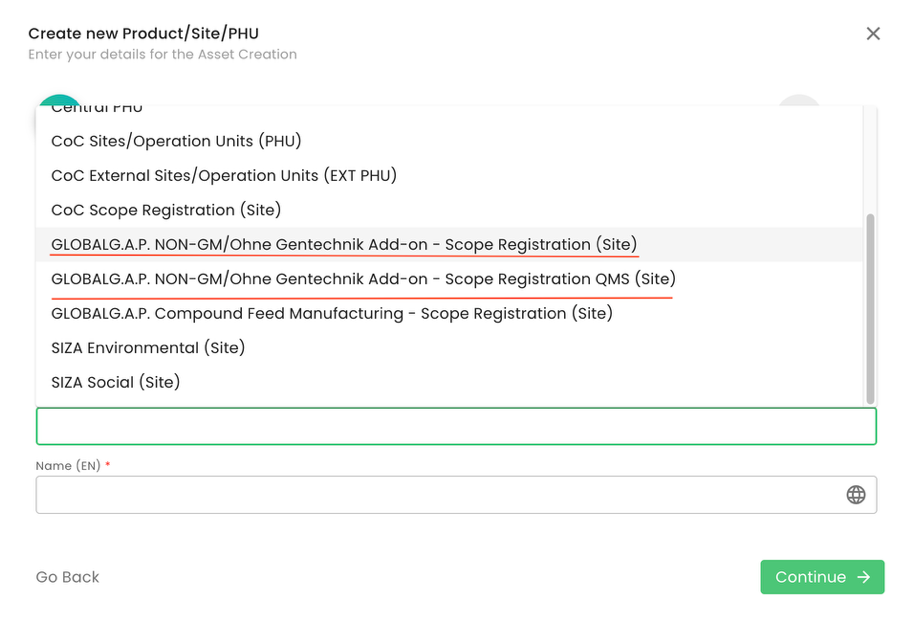
To initiate a GLOBALG.A.P. Non-GM/Ohne Gentechnik Add-on audit, we need to define our certification scope. To do so, we first must create the producer’s product scope site. We start by selecting the Type “Sites/PHU” and afterwards one of the applicable “NON-GM/Ohne Gentechnik Add-on” scope sites:
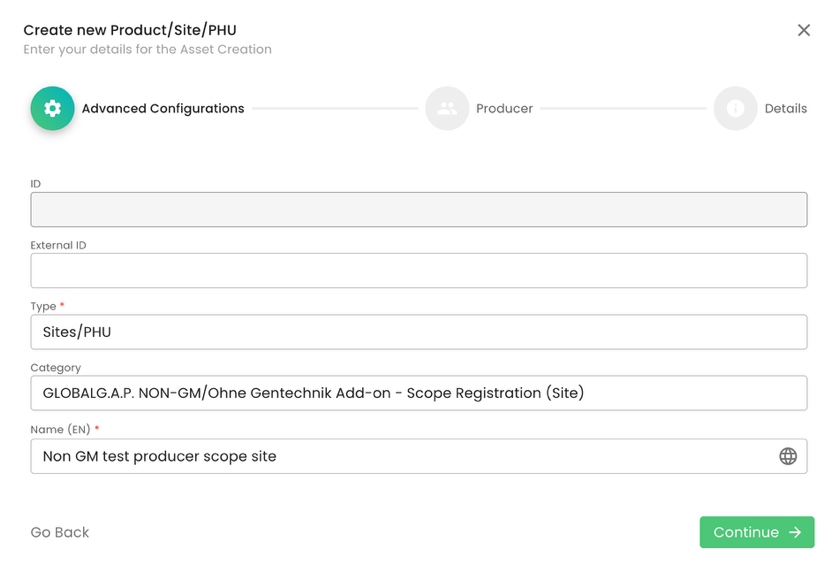
As per standard practice, proper site naming is essential:
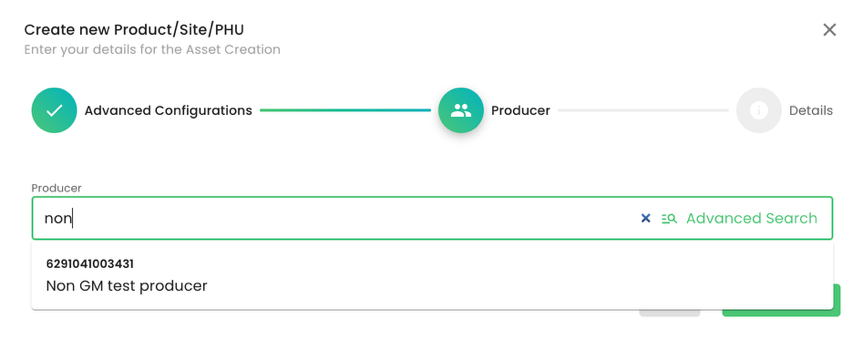
As always, we select the producer. In this example, it is the producer “Non GM test producer”:
Please note that adding a contact person is not required.
We do not add a Parent; we select our CB under organization and submit. As always, you can add tags or a description to the respective fields. Please note that for the scope site, an address is not required:
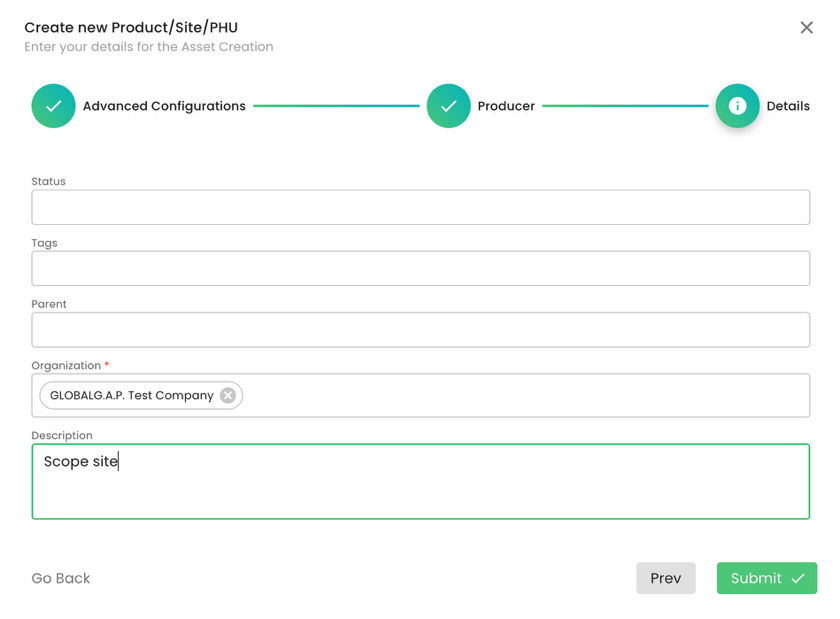
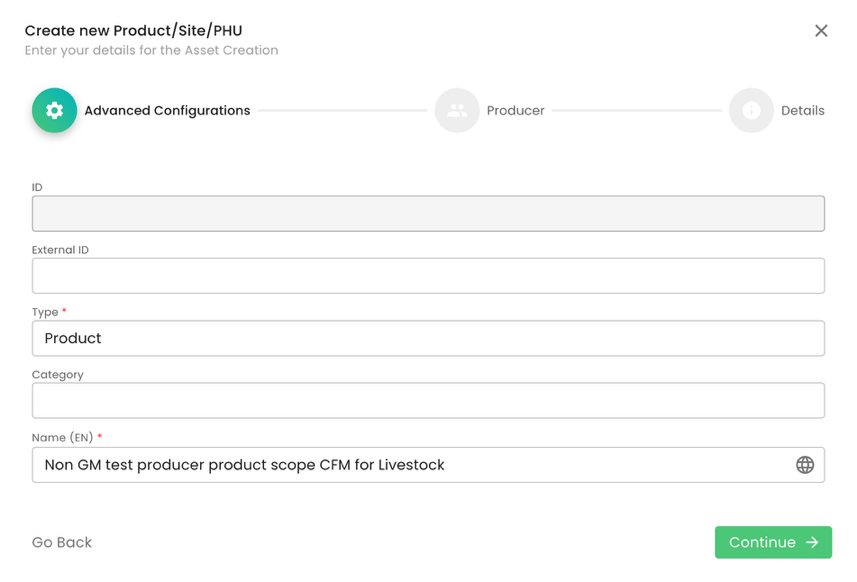
The next step is to add the certification scope (product) to the site above. As always, we select Type “Product” and properly name it. In our example, the certification scope is “CFM for Livestock”:
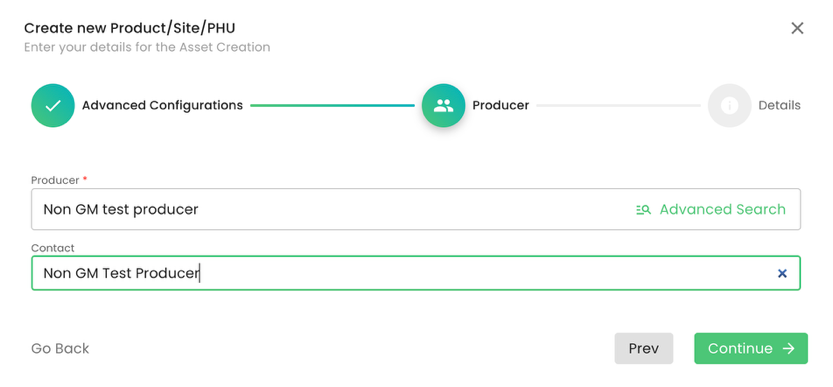
We select the producer:
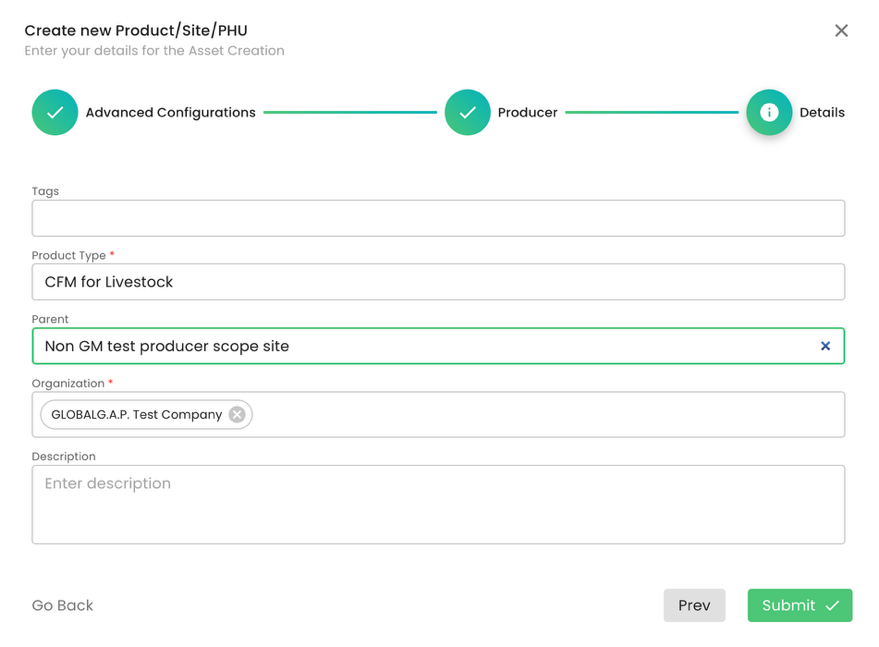
Under product type, for the FAP GLOBALG.A.P. NON-GM/Ohne Gentechnik Add-on, we have the following product options:
In our example, we will select the product “CFM for Livestock”, select under Parent the site we have created before, add our CB under Organization and press on submit:
In case the producer operates a PHU, we shall create one in the same way as for IFA. Proper naming is essential:
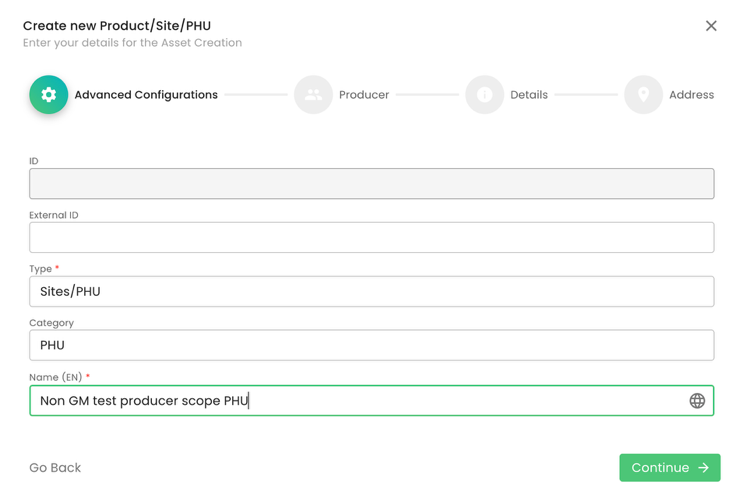
The next step is to add the product to the PHU we have just created:
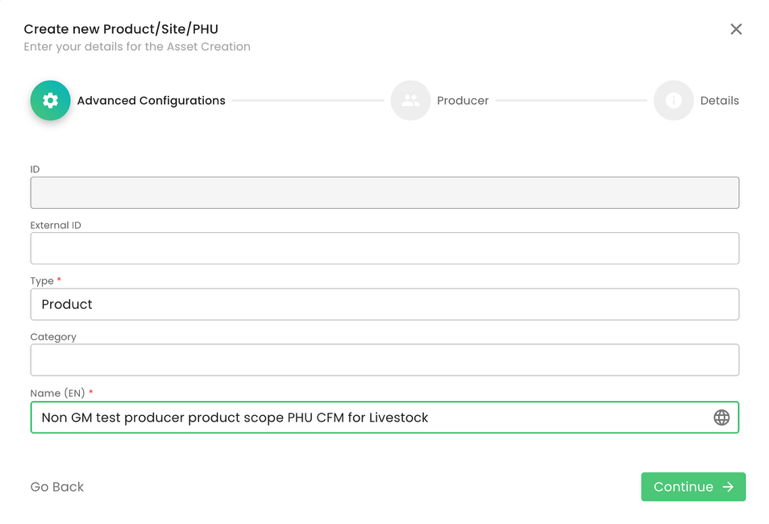
As always, we select the producer and contact person:
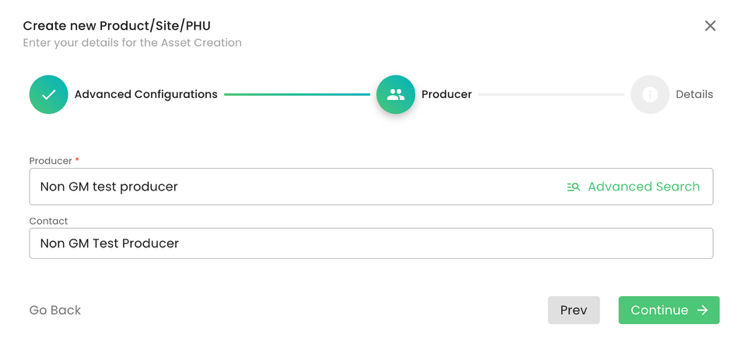
At the next step, we need to select the product “CFM for Livestock” and select the PHU:
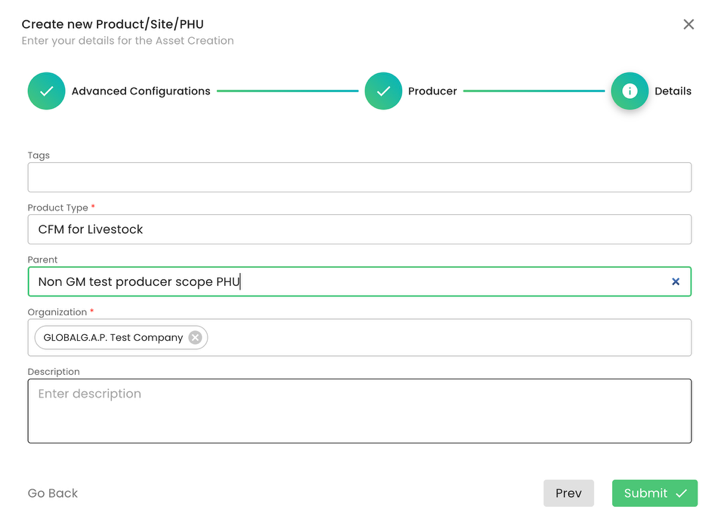
To summarise, in our example, we have created,
Note: The creation of the scope site and product scope needs to be done only for the first audit and can be used for all future audits.
After having finalised the creation of the scope, we can start the audit in the same way as always by selecting the Farm Assurance Product (FAP). First, we select the relevant FAP:
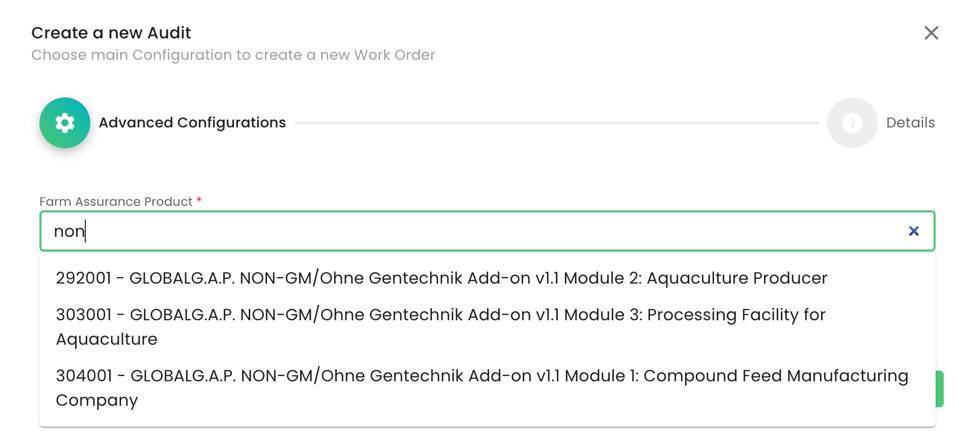
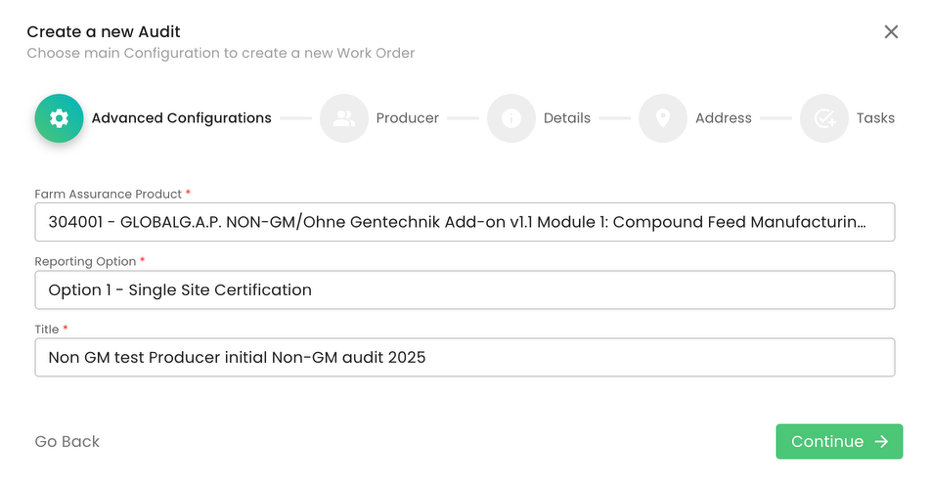
In our example, we selected Module 1 and as the reporting option “Option 1 - Single Site Certification” and named the audit:
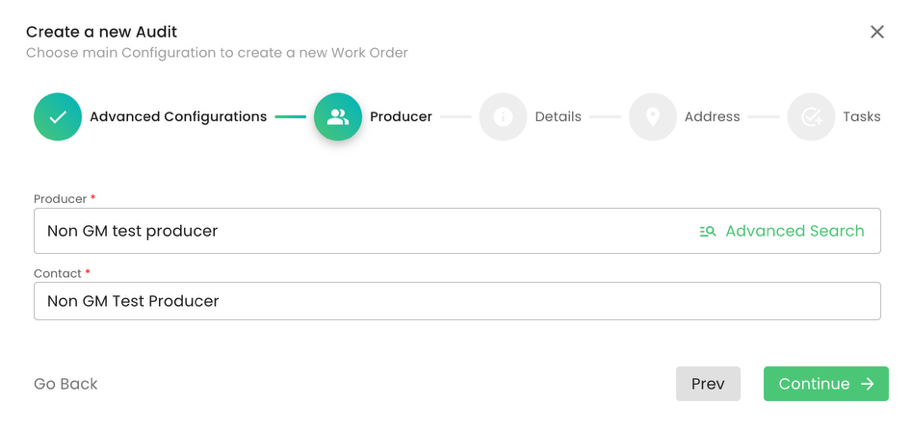
As always, we select the applicable producer:
Under Audit type, we select the applicable option. In our example, we selected “Initial certification audit”.
Please note that for all Non-GM modules under the Audit Product Category, we shall select “GLOBALG.A.P. NON-GM/Ohne Gentechnik scope”:
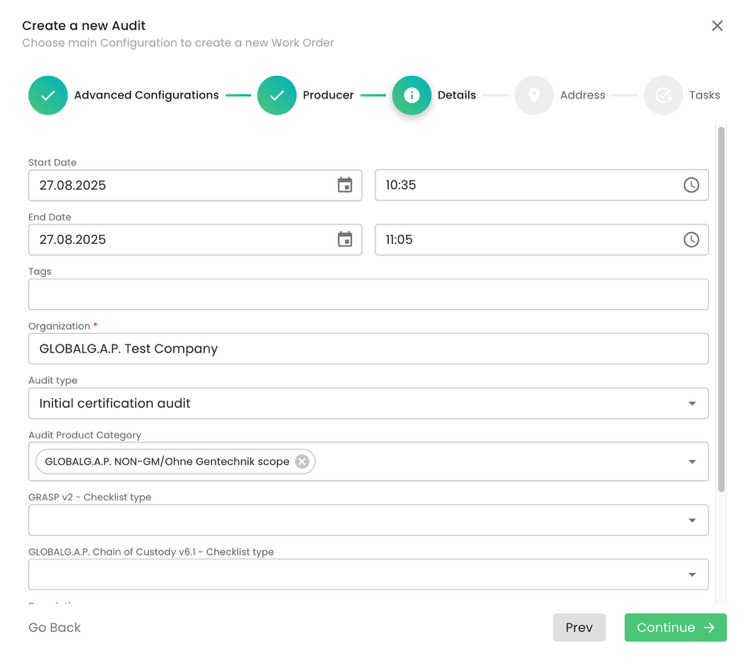
We click through the pop-up window by clicking “Continue” and "Submit" the audit.
The next step is to add the Scope information to our audit:
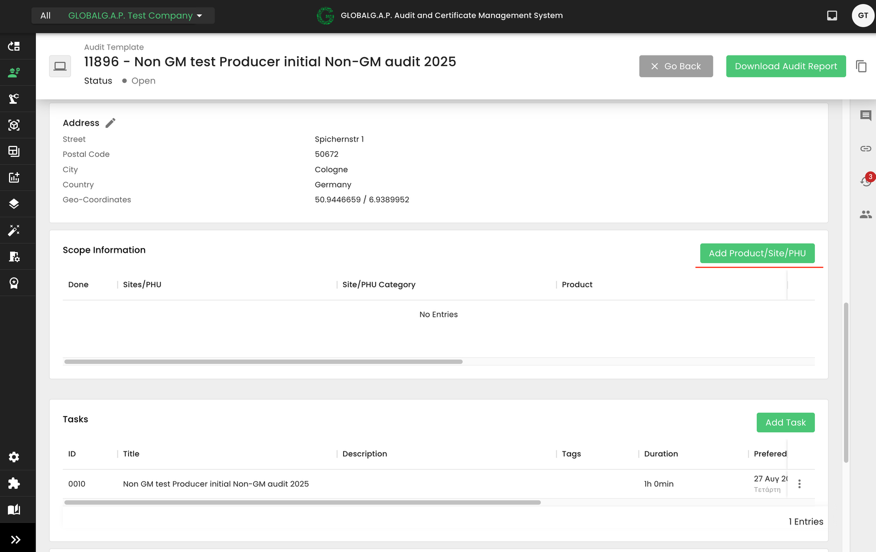
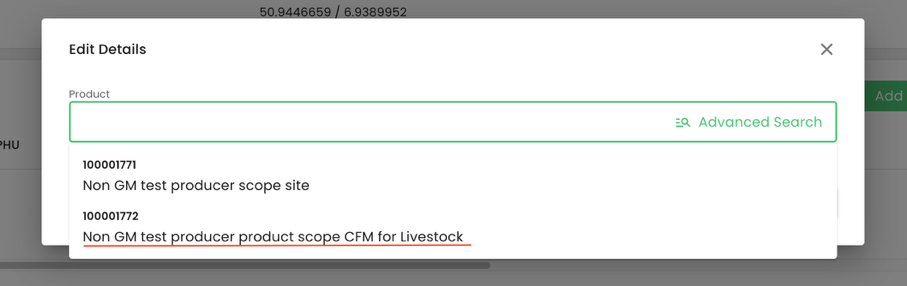
In our example, we are selecting the product we had previously named “Non GM test producer product scope CFM for Livestock”:
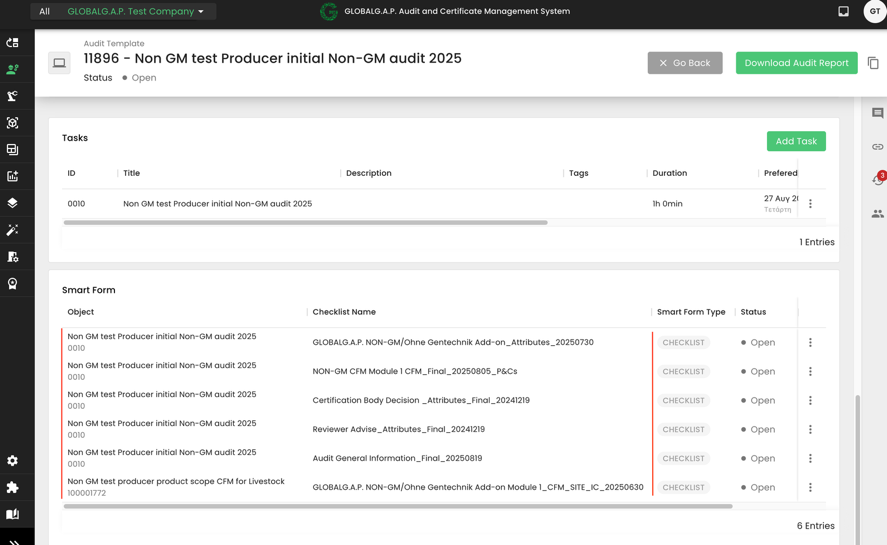
With this, the proper smart forms will be triggered:
After filling out the smart forms and finalizing the audit, the Letter of Conformance can be issued as explained in this wiki.
Note: in case the certification scope for GLOBALG.A.P. NON-GM/Ohne Gentechnik Add-on is “IFA for Aquaculture” and the producer is an Option 1 – Multiside with QMS, when creating the scope site, the option “GLOBALG.A.P. NON-GM/Ohne Gentechnik Add-on - Scope Registration QMS (Site)” must be selected:
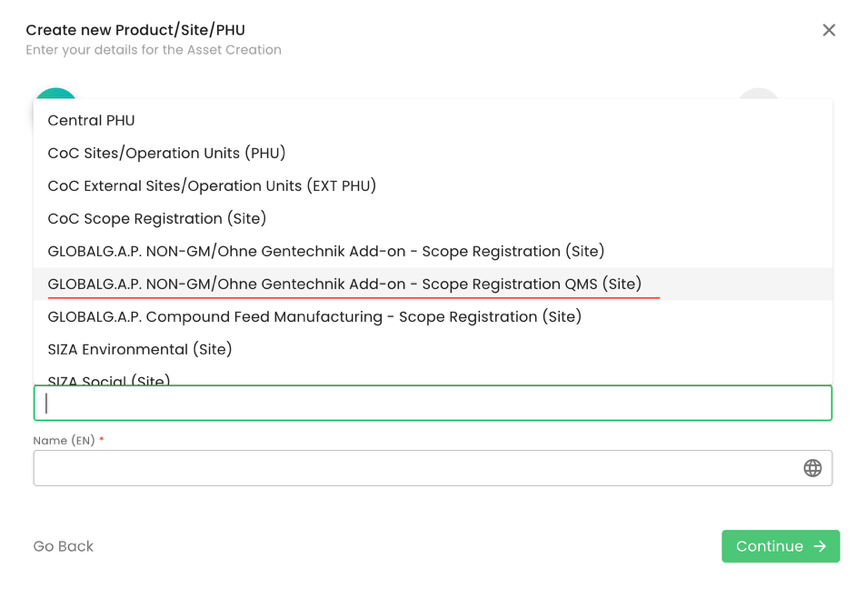
Note:
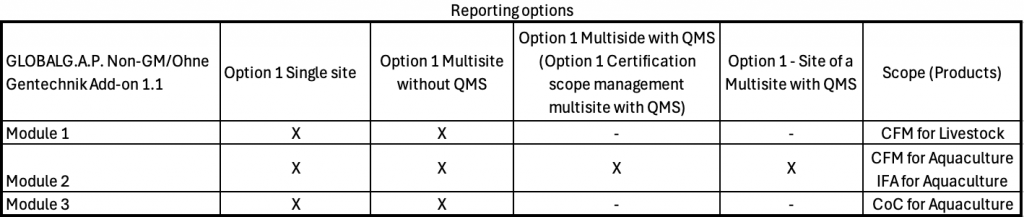
The available certification options per module for this Add-on can be seen below: